Axway eSubmissions. Fast. Secure. Integrated with Oracle Argus.
Accelerate submissions of events, applications, and critical data worldwide with a simple, secure, and globally compliant process.

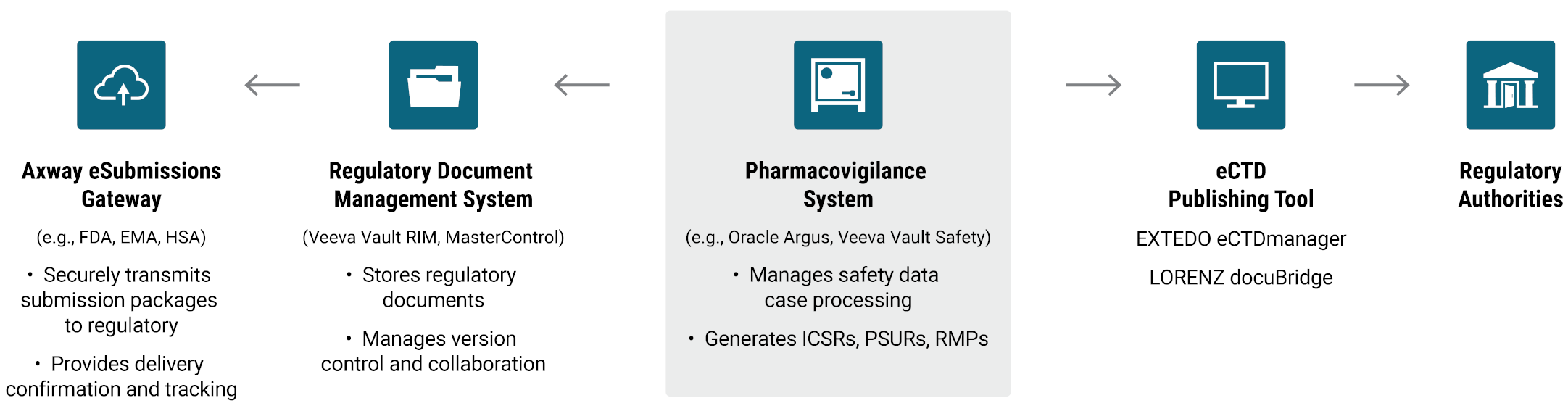
Electronic submissions management for pharmaceutical companies and CROs
Axway eSubmissions capabilities
Keep pace with regulators
When regulators change requirements, you need to keep up. Axway eSubmissions supports multiple protocols to ensure your connections are secure and that you consistently comply with regulatory authorities.
Manage movement to the cloud
Moving to the cloud? Axway eSubmissions allows you to deploy wherever you want. It provides secure, reliable access and control for your safety processes when you’re moving to the cloud.
Speed and simplify submissions
The next-gen eSubmissions gateway streamlines the process of preparing, making, and managing electronic submissions. Instantly locate and track your submissions across gateways, translators, and back-end systems.
Take a standardized approach
If you’re a Contract Research Organization (CRO) working with multiple pharma companies, you can create standardized practices for the eSubmissions process that apply across all of them.
Handle all protocol types in one process
Move beyond the typical limits of one-to-one relationships between protocols and instances of your safety database. eSubmissions gateway lets you manage all the protocols necessary to communicate locally and globally.
Talk to the FDA your way
Engage with the FDA in the way that’s easiest for you. With Axway eSubmissions, you can submit via the traditional eSubmissions, AS2, and/or API–whichever makes the most sense.